ZyCoV-D was created by Zydus Cadila, an Ahmedabad-based pharmaceutical company. The world’s first DNA immunisation against Covid-19 has been certified for emergency use by India’s pharma board. The three-dose ZyCoV-D vaccine, according to interim studies published by vaccine maker Cadila Healthcare, prevented clinical disease in 66 percent of those injected. The company expects to generate up to 120 million doses of India’s second home-grown vaccine per year. Previous DNA vaccines have been shown to work in animals but not in people. The ZyCoV-D vaccine is the first in a series of DNA vaccines being tested in clinical trials around the world for a variety of diseases.
Cadila Healthcare reported having conducted India’s largest vaccine clinical study to date, with 28,000 volunteers participating in over 50 locations. According to the business, this is the first time a Covid-19 immunisation has been tried in young people in India, with 1,000 children aged 12 to 18. In this age range, the jab was determined to be “safe and well-tolerated.”The crucial third round of clinical trials took place at the height of the virus’s lethal second wave. The vaccine’s “efficacy against mutant strains,” notably the extremely contagious Delta form, was reaffirmed as a result, according to the vaccine manufacturer.
The urgency of combatting COVID-19 has accelerated the development of vaccinations that utilise genetic technology, such as messenger RNA and DNA vaccines. Weiner claims that DNA and mRNA vaccines have been under research since the 1990s. DNA vaccines face a unique hurdle in that they must reach the cell nucleus, as opposed to mRNA vaccines, which just need to reach the cytoplasm, according to Jameel. Hence, DNA vaccines couldn’t produce powerful adaptive immunity in medical studies, which is why they have only been approved for animals until recently.
Source – BBC
The Working
DNA and RNA are the basic components of life. They are molecules that carry the genetic information passed down from generation to generation. A DNA vaccine, like other vaccinations, prepares the body’s immune system to fight the genuine virus once it is given. Most Covid-19 vaccines work by telling the body to make a fragment of the spike protein, which induces the immune system to build antibodies and teach itself how to fight the virus.
ZyCoV-D comprises plasmids, which contain circular strands of DNA that encode the SARS-CoV-2 spike protein as well as a promoter region for turning the gene on. When plasmids penetrate cells’ nuclei, they are transcribed to mRNA, which then travels to the cell’s main body, the cytoplasm, where it is translated into the spike protein. The immune system generates a response against the protein and produces immune cells that are specifically designed to clear future infections. Plasmids decay in weeks to months, but the immunity is preserved.
This is the world’s first human DNA immunisation against Covid-19. In the United States, for example, many DNA vaccines for use in animals have been licenced, including a vaccination for a sickness in horses and a skin cancer vaccine for dogs. Nonetheless, over 160 other DNA vaccines are being tested in human clinical trials in the United States. Most of the vaccines are used to treat pre-existing cancers, with one-third of them being used to treat HIV.
ZyCov-D is also the first needle-free Covid-19 vaccination in India. It’s administered with a needle-free injector that uses a small stream of fluid to enter the skin and deliver the injection to the proper location. ZyCoV-D is deposited beneath the epidermis rather than deep within muscle tissue. Immune cells populate the area beneath the skin, which eat and digest foreign items such as vaccine particles. The vaccination is given by a needle-free device that is placed against the skin and creates a thin, high-pressure stream of fluid that punctures the skin and is less unpleasant than an injection.
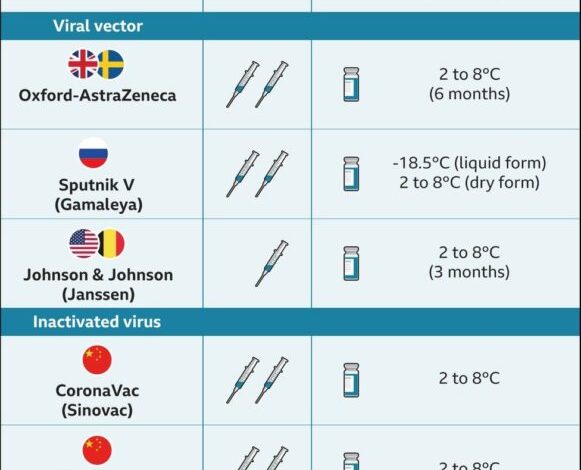
DNA immunizations are very inexpensive, safe, and stable, according to scientists. Higher temperatures, ranging from 2 to 8 degrees Celsius, can also be used to preserve them. Cadila Healthcare claims that its immunisation has shown “great stability” for at least three months at 25 degrees Celsius, making it easy to transport and store.
Source – News On Air
The Drawbacks
Previously, DNA vaccines failed for infectious diseases in humans. The issue, according to Dr. Kang, is that they perform well in animals but don’t provide the same level of immune response protection in humans. According to Dr. Kang, the problem was to get the plasmid DNA into the human cell and have it produce a long-lasting immunological response.
The mRNA vaccines developed by Pfizer and Moderna, that use messenger RNA (a molecule) to synthesize proteins, do not need to reach the cell nucleus to be functional. They have a better efficacy and are more likely to produce longer-lasting protection. Earlier this year, the ZyCoV-D studies in India were undertaken when the Delta variation of SARS-CoV-2 was the prevalent variant in circulation, but earlier mRNA vaccine trials were conducted when less transmissible variants were circulating.
ZyCoV-D, which is administered through the skin rather than an injection, was proven to be 67 percent protective against symptomatic COVID-19 in clinical trials, and it will most likely be utilised in India this month. Although the vaccine’s efficacy isn’t as good as many other COVID-19 vaccinations, the fact that it’s a DNA vaccine, according to researchers, is noteworthy.
Another downside of ZyCoV-D is that it requires three doses, whereas the other two Indian candidates only require two. This is expected to make providing the vaccination during the current epidemic more difficult logistically. The vaccine manufacturer claims to be investigating the possibility of a two-dose vaccine.
Because no late-stage study findings have yet been released, some experts have challenged a lack of transparency in the approval process. The trial is still ongoing, according to Zydus Cadila, and the entire analysis will be published soon. The first doses will be given out in India in September, and the company expects to create up to 50 million doses by early next year.
Source – Monash Council
Other DNA Vaccines
Other DNA vaccines against COVID-19 are being developed, with a range of antigens and delivery systems (see ‘DNA vaccines in clinical trials’). Two have entered late-stage trials: one by AnGes, a Japanese business based in Osaka, and the other by Inovio Pharmaceuticals, based in Plymouth Meeting, Pennsylvania, which Weiner assisted in developing. Inovio is a vaccination that is administered beneath the skin using a device that employs short electric pulses to produce pores in the cells that allow the vaccine to flow through.
Over a half-dozen COVID-19 DNA vaccines are in preclinical studies, such as one developed by the South Korean biotech company GeneOne Life Science in Seoul the other by the Thai firm BioNet in Bangkok, in which Richmond is involved.In Australia, a phase I trial for this vaccine is underway.
However, Richmond anticipates that many more DNA vaccines will be developed, focusing on diseases for which there are presently no vaccines, such as cytomegalovirus, which can be transmitted to new-borns during pregnancy, and respiratory syncytial virus. For influenza, human papillomavirus, HIV, and Zika, DNA vaccines are being tested or created.
DNA vaccines can hold a great amount of data, allowing them to encode large, complex proteins or even numerous proteins. That, according to Weiner, makes them promise as anti-cancer vaccinations, a prospect he is investigating in his own research.
Written by- Niki Shah
Edited by- Ginia Chatterjee
The post First Covid DNA vaccine in India appeared first on The Economic Transcript.





